ABOVE: Scientists use phage display to generate monoclonal antibodies. © istock.com, mirror-images
When recombinant protein expression was introduced in 1977, screening clones was a laborious and time-consuming process.1-3 While on a sabbatical at Duke University, George Smith, a biochemist at University of Missouri, reasoned that he could improve this process by leveraging a coat protein, called pIII, from the filamentous bacteriophage that he studied. He tested his initial design with two Duke University biochemists, Robert Webster and Paul Modrich. Their collaboration birthed phage display, a combinatorial marvel that Smith referred to in his Nobel lecture as “simple evolution in a Petri dish.” It completely changed the field of recombinant protein biology.
The first phage display library
In the early and mid-1980s, scientists used phages to express recombinant proteins. The protein was retained inside the virions, so screening required growing viral plaques, creating a stamp of these on a membrane, treating the membrane with antibody, and then mapping antibody labels on the membrane back to the exact viral plaques on the Petri plate.
Smith’s vision for improving recombinant protein analysis was to express a foreign protein at one end of pIII and then select for it using an antibody affixed to a surface. In this way, scientists could retain only positive clones and immediately use their selected phage to generate more phages. This made screening higher throughput, as researchers could screen thousands or even millions of clones simultaneously.
It completely opened the door to making fully human antibodies as therapeutics.
—John McCafferty, Cambridge Institute of Therapeutic Immunology and Infectious Disease
Additionally, because the protein would be bound to the bacteriophage with the inserted DNA, the product and instructions for it were obtained at the same time as a genotype-phenotype linkage. “You can then sequence that particular phage clone, and it will tell you the nucleotide sequence and therefore, the amino acid sequence of the peptide displayed on its surface,” said Jamie Scott, who joined Smith’s lab as a postdoctoral fellow in the late 1980s and is now a professor emeritus at Simon Fraser University.
When Smith returned to his lab, he explored this method further, and in 1985, published his work on what he called filamentous phage display.4 In subsequent years, Smith and his team developed a peptide library in which five to six amino acid residues in a peptide sequence were randomized.5 In this work, they demonstrated the value of phage display in selecting for high-binding peptides through a series of affinity purification steps, where bound phages were eluted, amplified in a bacterial host, and then reintroduced to their target ligand to select for binders. These libraries allowed for screening potentially billions of random epitopes simultaneously.
While Smith and his colleagues’ peptide libraries demonstrated the potential of phage display, the sequences were randomly generated. Scientists did not have control over the amino acid frequencies, and because of redundancies in amino acids, could have overrepresentation of some residues. To improve control over the amino acids used and circumvent the introduction of stop codons, subsequent groups developed methods to synthesize codons individually.6 These presynthesized codons can be mixed at specific percentages to yield a library with the characteristics researchers are most interested in, such as polarity and binding affinities.
“It’s not random,” said Anthony Kossiakoff, a protein engineer at the University of Chicago who studies protein structure-function relationships. “There’s a lot of planning and designing for how you can best utilize what you got.” Proficient structural biologists consider more than the library.
“The biggest thing about any of these selections is the quality of the antigen,” Kossiakoff said. He explained that for phage display to yield usable products, teams using the method need to determine the stability of the antigen and ensure that it is in the desired conformation.
Lastly, to select for high-affinity proteins, researchers must develop adequate stringency conditions in their affinity purification steps. This may include decreasing the concentration of ligand, introducing competing enzymes, screening against ligands in other conformations, or blocking potential binding sites of the target ligand during the maturation step.7
At the end, because of the genotype-phenotype linkage, a researcher can extract the protein’s genetic sequence for a variety of downstream purposes.
PHAGE DISPLAY LIBRARY GENERATION Scientists insert variable DNA sequences coding for their proteins of interest into plasmids that carry a phage coat protein gene, an antibiotic resistance gene, and a packaging signal. Then they transform the plasmids into a bacterial vector and infect it with a helper phage that supplies the other necessary viral proteins. This generates the phage library, where each virion expresses versions of the protein of interest on its coat protein and carries its genetic sequence in its genome. |
AFFINITY PURIFICATION In the next step, researchers isolate the phages expressing surface proteins using a surface ligand binding assay. The final eluted phages may bear a mix of different surface protein epitopes. |
PHAGE AMPLIFICATION In the amplification step, researchers propagate the eluted phages in a bacterial culture, and may run additional rounds of affinity purification. |
CLONE ISOLATION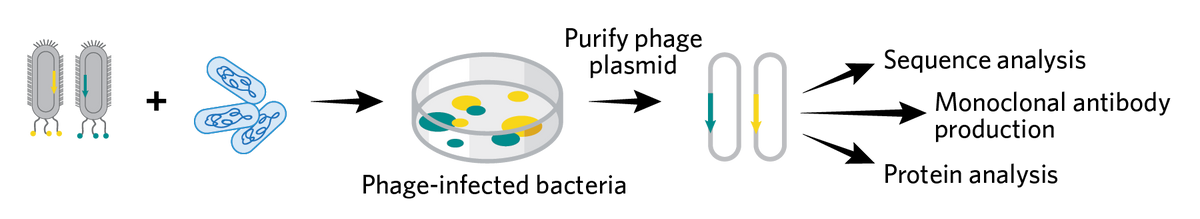 Researchers infect bacteria with the selected phages and culture them on plates containing antibiotics. In the final step, they pick resistant colonies to isolate the plasmids, which are then sequenced and cloned into the desired protein production vectors for various applications. |
Opening the door to phage display antibodies
This linkage of the instructions with the binding product, coupled with the ability to evaluate huge volumes of candidates, became an attractive model for many research applications, but particularly for antibody research and production.
While Smith’s method generated random peptide sequences and tested them against known antibodies, other groups considered alternative approaches. “Wouldn’t it be really cool if we could do that the other way around?” said John McCafferty, currently a biologist at the Cambridge Institute of Therapeutic Immunology and Infectious Disease and chief executive officer of Maxion Therapeutics. He and his postdoctoral mentor, Greg Winter, a molecular biologist currently at the Medical Research Council Laboratory of Molecular Biology, used phage display to produce antibodies against known targets.
By cloning the variable regions of the heavy and light chains of antibodies from immunized blood donors together on a single coding region, the two produced a fusion protein with all of the antigen binding properties of a full antibody, which they called single chain Fv (scFv). This scFv was expressed by the bacteriophage and could be affinity selected analogously to Smith’s peptides by using the immobilized peptide as a binding target for the antibodies. From the inception of this idea to the publication of the first successful antibody took one year8—“A feat that I’ve tried to repeat ever since without success,” McCafferty said.
It’s an extremely powerful technique to do all kinds of things.
—Anthony Kossiakoff, University of Chicago
Producing monoclonal antibodies was previously possible using hybridoma methods.9 However, these methods could be laborious and take months to immunize animals, isolate and culture B cells that were fused to immortalized cells, and then screen them. With phage display, it was possible to isolate the genetic material from B cells from immunized animals or humans and immediately begin amplifying it in bacteriophages. Not only was this faster, it also simplified the process of studying the genetic sequence of these antibodies. Additionally, it alleviated the problems of hybridoma technology for producing animal antibodies that had to be humanized. The binding sequences identified through phage display could be isolated, reinserted into an existing human IgG backbone, and expressed in bacterial or mammalian cells. “It completely opened the door to making fully human antibodies as therapeutics,” McCafferty said.
This method led to the development of the world’s first antibody produced by phage display, the anti-TNF antibody adalimumab, more commonly known as Humira, which was produced by McCafferty’s and Winter’s company Cambridge Antibody Technology with the help of Baden Aniline and Soda Factory (BASF).
Coupled with next-generation sequencing, phage display allows for the rapid production and assessment of antibodies against an array of targets, including those that would otherwise be impossible to produce in a human system. “Because in a synthetic library, it has no basis for tolerance,” said Scott.
An extremely powerful technique
Phage display, like most technologies, has evolved. In the world of antibody production, researchers explored display vehicles such as nontraditional viruses and bacteria and also used eukaryotic cells, such as yeast and mammalian cell lines.10,11
“In some ways, a phage display system is a bit of a black box,” McCafferty said. “You’ve got your library; you’ve got your antigen; you’ve been through a process; and out comes stuff at the other end.” While one can easily find a slew of binding candidates, it’s often more than can be easily or desirably assessed for detailed binding affinity or cross-recognition with other potential targets.
While yeast and mammalian cell display methods do not offer the scale of library potential that phages do, these approaches are compatible with flow cytometry and cell-sorting applications, which allow researchers to explore far more of their protein-expressing clones and their biophysical properties.
Researchers also use alternative backbones for their peptides beyond the traditional immunoglobulin scaffolds. These include small proteins or even domains of proteins, such as fibronectin binding domain, which can support the introduction of variable peptide regions.12 These new scaffolds can be smaller than immunoglobulin and overcome structural limitations, such as the immunoglobin’s dual-chain design, a double cysteine bond, and necessary post-translational modifications.13,14
Modified selection methods also expand the capacity to select displayed proteins with desired characteristics. This includes in vivo selection, where candidates are delivered to a model animal and their organ distribution is assessed, and whole cell culture, where the library can be screened against multiple cell types to determine receptor recognition.15-17
Beyond antibodies, researchers use phage display to explore and manipulate protein interactions, either to study protein functions or to apply toward novel drug discovery.18,19 “You can ask questions that are very outside the box about the energetics of interfaces and understand, as evolution has put these things together, what was really important, and why it’s important and conserved,” Kossiakoff said.
Phage display was used to identify rare or even unnatural peptides for cancer therapeutics and B cell epitopes in disease and to screen against allergens to characterize them or chemical compounds to study their mechanism of action.20-23 It’s also being explored for identification and production of antivenom antibodies, some of which are cross protective against venom from multiple snake genera.24,25 “It’s an extremely powerful technique to do all kinds of things,” Kossiakoff said.
References
- Itakura K, et al. Expression in Escherichia coli of a chemically synthesized gene for the hormone somatostatin. Science. 1977;198(4321):1056-1603
- Mierendorf RC, et al. Gene isolation by screening λgt11 libraries with antibodies. Methods Enzymol. 1987; 152: 458-469
- Young RA & Davis RW. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci. 1983;80(5):1194-1198
- Smith GP. Filamentous fusion phage: novel expression vectors that display cloned antigens on the virion surface. Science. 1985;228(4705):1315-1317
- Scott JK & Smith GP. Searching for peptide ligands with an epitope library. Science. 1990;249(4967):386-390
- Virnekas B, et al. Trinucleotide phosphoramitides: ideal reagents for the synthesis of mixed oligonucleotides for random mutagenesis. Nucleic Acids Res. 1994;22(25):5600-5607
- Pande J, et al. Phage display: concept, innovations, applications, and future. Biotechnol Adv. 2010;28(6):849-858
- McCafferty J, et al. Phage antibodies: filamentous phage displaying antibody variable domains. Nature. 1990;348:552-554
- Mitra S & Tomar PC. Hybridoma technology; advancements, clinical significance, and future aspects. J Genet Eng Biotechnol. 2021;19(159)
- McMahon C, et al. Yeast surface display platform for rapid discovery of conformationally selective nanbodies. Nat Struct Mol Biol. 2018;25:289-296
- Robertson N, et al. Development of a novel mammalian display system for selection of antibodies against membrane proteins. J Biol Chem. 2020;295(52):18436-18448
- Gilbreth RN & Koide S. Structural insights for engineering binding proteins based on non-antibody scaffolds. Curr Opin Struct Biol. 2012;22(4):413-420
- Binz HK & Plückthun A. Engineered proteins as specific binding reagents. Curr Opin Struct Biol. 2005;16(4):459-469
- Skerra A. Imitating the humoral immune response. Curr Opin Chem Biol. 2003;7(6):683-693
- Kolonin MG, et al. Reversal of obesity by targeted ablation of adipose tissue. Nat Med. 2004;10:625-632
- Pasqualini R & Ruoslahti E. Organ targeting in vivo using phage display peptide libraries.Nature. 1996;380:364-366
- Barry MA, et al. Toward cell-targeting gen therapy vectors:selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med. 1996;2:299-305
- Sidhu SS, et al. Exploring protein-protein interactions with phage display. Chembiochem. 2003;4(1):14-25
- Krumpe LRH & Mori T. Potential of phage-display peptide library technology to identify functional targeting proteins. Expert Opin Drug Discov. 2007;2(4):525-537
- Brown KC. Peptidic Tumor Targeting Agents: The Road from Phage Display Peptide Selections to Clinical Applications. Curr Pharm Des. 2010;16(9):1040-1054
- Dybwad A, et al. Identification of new B cell epitopes in the sera of rheumatoid arthritis patients using a random nanopeptide phage library. Eur J Immunol. 1993;23(12)3189-3193
- Rhyner C, et al. Cloning allergens via phage display.Methods. 2004;32(3)212-218
- Van Dorst B, et al. cDNA phage display as a novel tool to screen for cellular targets of chemical compounds. Toxicol in Vitro. 2010;24(5):1435-1440
- Ahmadi S, et al. An in vitro methodology for discovering broadly-neutralizing monoclonal antibodies. Sci Rep. 2020;10:10765
- Ledsgaard L, et al. Discovery and optimization of a broadly-neutralizing human monoclonal antibody against long-chain-α-neurotoxins from snakes. Nat Commun. 2023;14:682